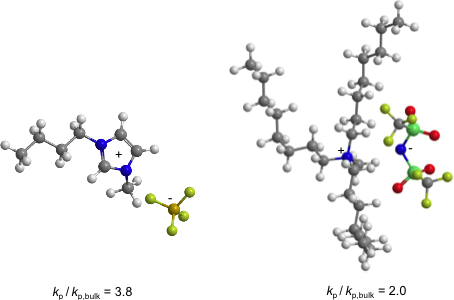
Propagation rate coefficients, kp, of methyl methacrylate, MMA, homopolymerizations in ionic liquids (ILs) with widely varying structures and physical properties were determined applying the socalled PLP-SEC technique, which combines pulse-laser initiation of the polymerization (PLP) with molecular weight analysis via size-exclusion chromatography (SEC). For all ILs a strong enhancement of kp and a decrease in the activation energy of k p compared to the bulk system were observed. Depending on the IL structure, the increase in kp is between a factor of 2 and 4. Both the cation and the anion are responsible for the enhancement of kp. The smaller the ions of the ILs, the stronger the enhancement of kp. Investigations into the solvent properties of ILs using a solvatochromic dye and infrared spectroscopy suggest that electron pair acceptor-electron pair donator interactions and nonspecific (Coulomb) interactions contribute strongly to the solvent influence, whereas contributions from H bonding are negligible. The knowledge of normalized polarity values of the ILs allows for a rough estimate of the IL-induced variation of kp.